FDA Approves Expanded Indication for Banamine Transdermal
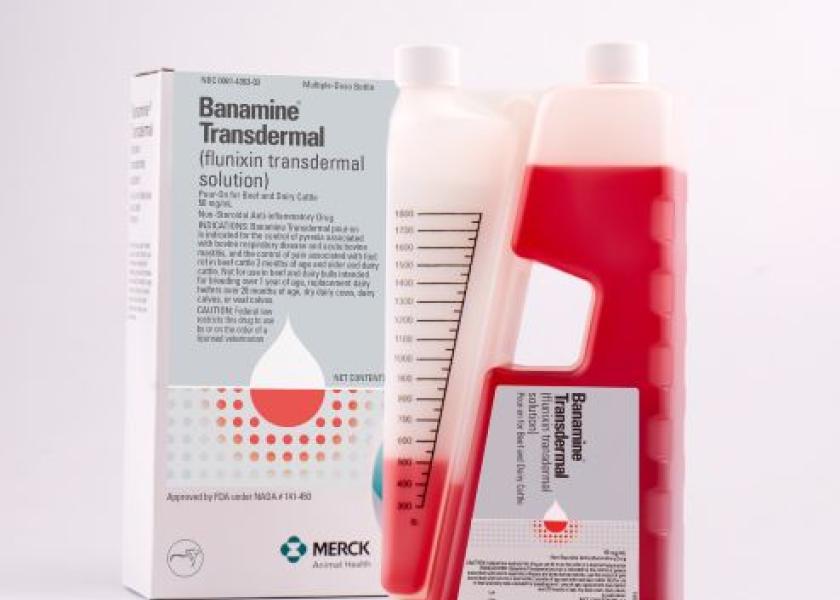
The U.S. Food and Drug Administration (FDA) has approved an expanded indication for Banamine Transdermal (flunixin transdermal solution). The new indication is for the control of pyrexia (fever) due to acute mastitis with a short milk withhold of 48 hours.
“This new indication means Banamine Transdermal can be given with confidence to lactating cows,” says Scott Nordstrom, DVM, director of livestock innovation and discovery for Merck Animal Health. “With simple, pour-on administration along the animal’s back, Banamine Transdermal saves time and labor costs while getting cows back into the milking string fast.”
In a multi-site field study, 95% of dairy cows with acute mastitis had a reduction in fever of 2° F or more six hours after treatment. That compared to 35% of untreated controls.1
Nordstrom says studies show a single dose of the product is absorbed into the bloodstream within minutes2 and has a long duration of activity at the site of inflammation3, giving cattle a good opportunity to recover quickly and return to productivity.
Introduced in 2018, Banamine Transdermal is the first and only FDA-approved product for pain control in a food-producing animal, and the first and only non-steroidal, anti-inflammatory (NSAID) cattle product available as a pour-on. It is a prescription product labeled for control of fever associated with bovine respiratory disease and acute mastitis, and for control of pain associated with foot rot.
Nordstrom says the product is easy to administer and eliminates the time-consuming and stressful treatment process associated with intravenous (IV) administration, which is the administration route of previous NSAIDs.
Workers can easily administer Banamine Transdermal without extensive training, Nordstrom says. The pre-calibrated packaging and red-colored solution help ensure the correct dose is given every time. The unique bottle design makes it simple to apply topically on dry skin in a narrow strip down the animal’s midline from the withers to the tail head.
References:
1. Evaluation of the efficacy and safety of flunixin transdermal solution for the control of pyrexia and/or inflammation associated with naturally occurring bovine mastitis (Study No. S12074-00). 2014.
2. Data on file: EX-05331-00.
3. Lees P, Higgins AJ. Flunixin inhibits prostaglandin E2 production in equine inflammation. Res Vet Sci. 1984; 37:347-349.